Prevalence of CTD-ILDs
Pulmonary fibrosis is a critical threat across a broad range of connective tissue disease-associated interstitial lung diseases (CTD-ILDs)1–5

INTERSTITIAL LUNG DISEASE (ILD) IS A COMMON EARLY MANIFESTATION OF CTDs AND SHARES COMMON PATHOGENIC PATHWAYS TO FIBROSIS1–7
ILDs make up a diverse group of more than 200 heterogeneous lung disorders, mostly classified as rare or only infrequently seen in clinical practice8–10
While some ILDs are idiopathic, others manifest as a result of environmental exposure to antigens or as a pulmonary complication of an underlying CTD11
In CTD-ILDs, pulmonary fibrosis is characterised by the often chronic and irreversible scarring of lung tissue,12,13and is a key driver of irreversible lung damage and early mortality in CTDs.1–5,14
What is the prevalence of ILD in CTDs?
Prevalence estimates for different CTDs and CTD-ILDs vary between different studies. Prevalence figures shown below are based on a range of different estimates from different studies15–40
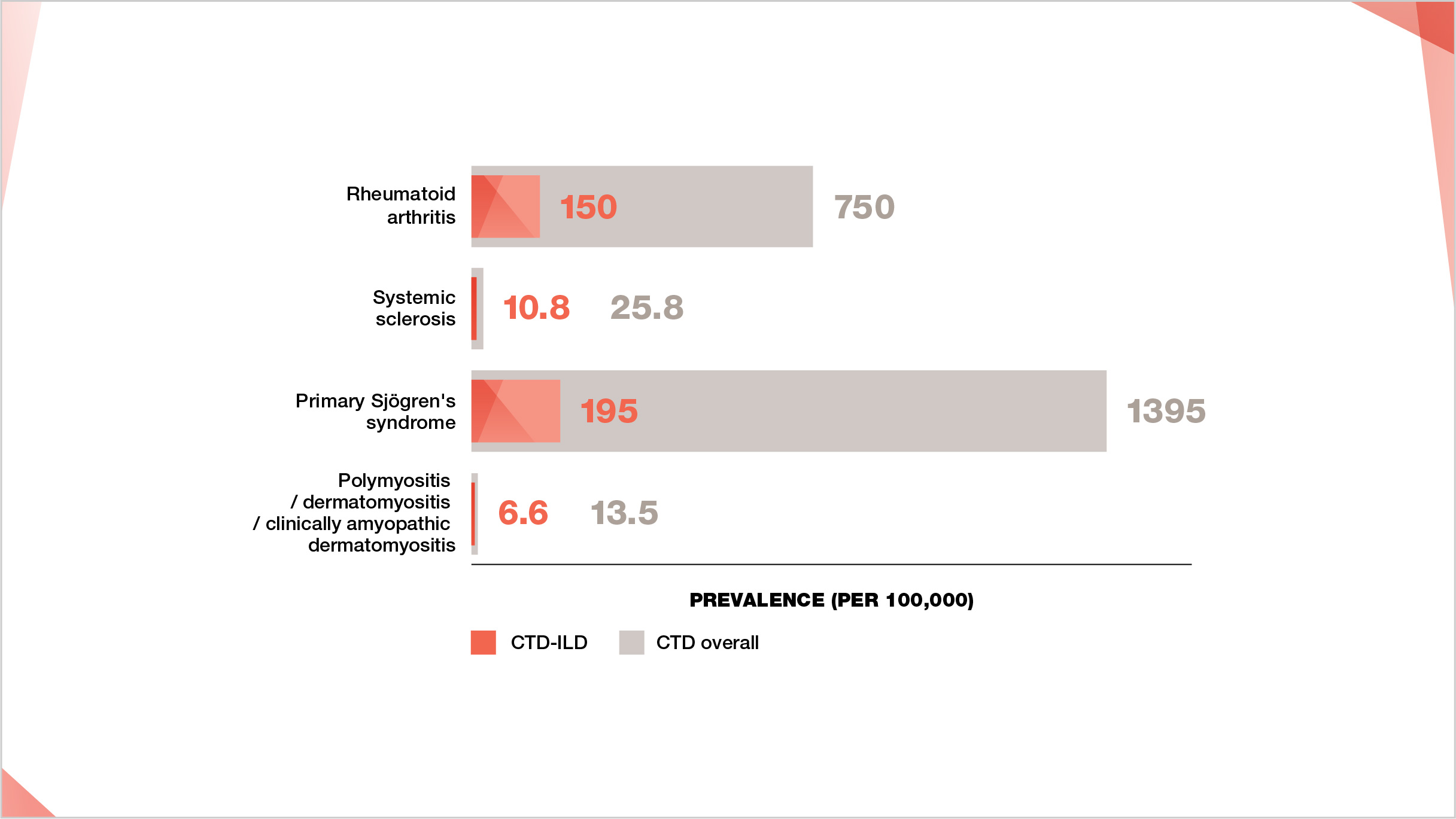
Prevalence of CTDs and CTD-ILDs per 100,000.
Prevalence figures sourced as midpoint values from ranges for CTD and CTD-ILD prevalence as follows: RA prevalence 500–1000 per 100,000;15–17 RA-ILD prevalence 10%–30% of RA.18-22 SSc prevalence 7.2–44.3 per 100,000;23 SSc-ILD prevalence 42% of SSc.24 primary Sjögren’s syndrome prevalence 90–2700 per 100,000;25,26 primary Sjögren’s syndrome-ILD prevalence 8%–20% of primary Sjögren’s syndrome.27-30 PM/DM prevalence 5–22 per 100,000;31,32 PM/DM/CADM-ILD prevalence 20%–78% of PM/DM/CADM.33-40
CTD-ILDs are an important subgroup of ILDs10
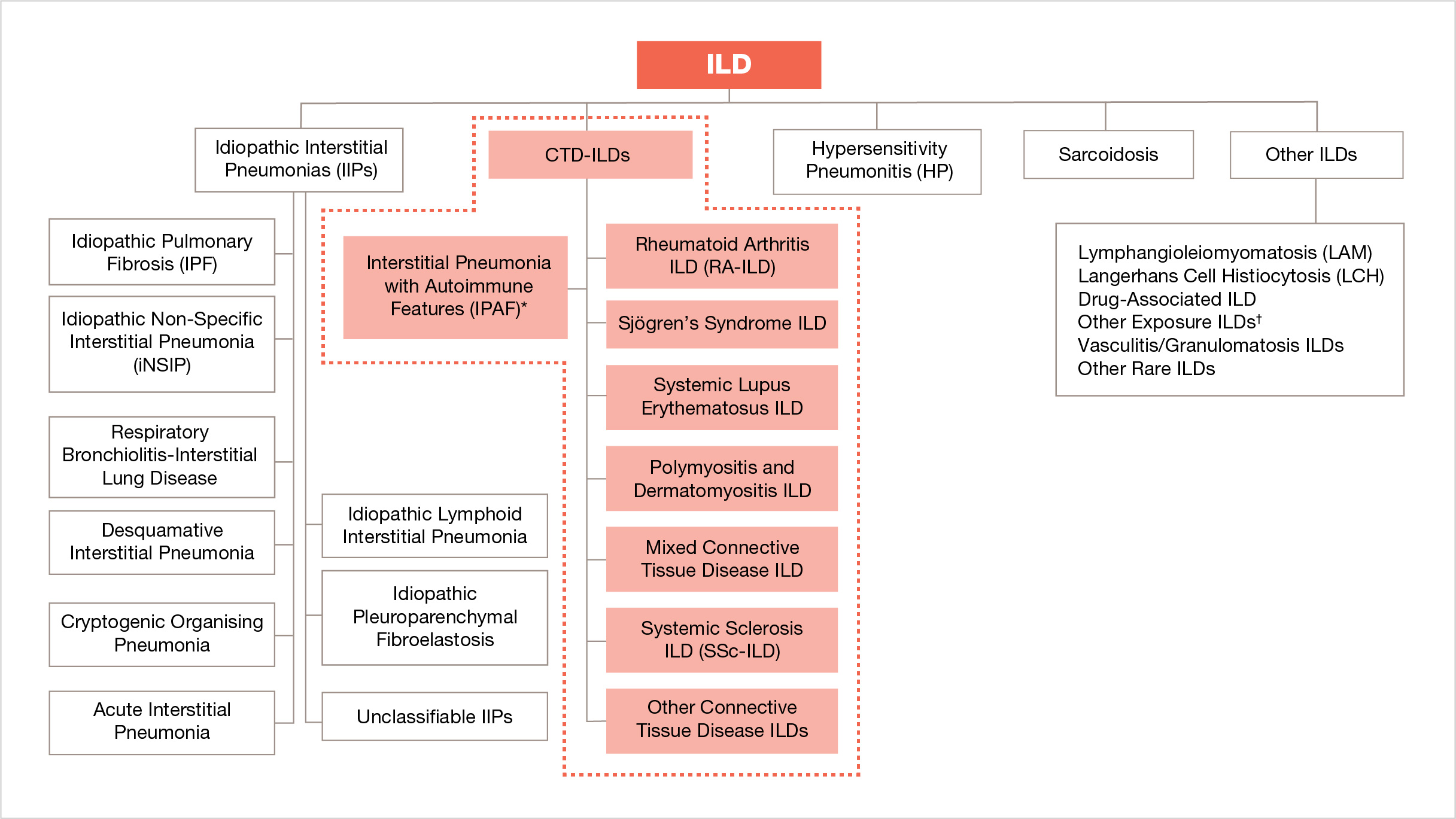
* Not an estimated clinical diagnosis
† For example: asbestosis, silicosis.
Adapted from: Cottin V, et al. Eur Respir Rev. 2018;27:180076.



ILD develops in 8%–20% of patients with primary Sjögren’s syndrome27-30
Find out more about the prevalence of primary Sjögren’s syndrome-ILD
WHY IS EARLY DIAGNOSIS OF ILD IMPORTANT?
ILD often develops early in the course of a CTD, and may even be the first manifestation of a previously undiagnosed or unrecognized CTD.1,2,5,6 ILD in CTDs may be subclinical in nature (presenting without symptoms),7 be chronically progressive, or even present in a life-threatening manner.42
Acute exacerbation of ILD, characterized by rapid respiratory deterioration with severe hypoxemia, can occur in patients with CTD-ILDs at any point during the course of disease.43–47 Based on patients with IPF, acute exacerbation of ILD is most likely triggered by an acute event, such as infection.48 Post-exacerbation hospital mortality in patients with CTD-ILDs is reported to range from 50%–100%.43
What could fibrotic ILD mean for your patients with CTDs?

Increased mortality rates
Acute exacerbation of ILD in CTD-ILDs

CTD-ILD patient cases
Footnotes
CTD, connective tissue disease; CTD-ILD, connective tissue disease-associated interstitial lung disease; IIP, idiopathic interstitial pneumonia; ILD, interstitial lung disease; HP, hypersensitivity pneumonitis; IPAF, interstitial pneumonitis with autoimmune features; IPF, idiopathic pulmonary fibrosis; LAM, lymphangioleiomyomatosis; LCH, Langerhans cell histiocytosis; NSIP, non-specific interstitial pneumonia; RA, rheumatoid arthritis; RA-ILD, rheumatoid arthritis-associated interstitial lung disease; SSc, systemic sclerosis; SSc-ILD, systemic sclerosis-associated interstitial lung disease.
-
Fischer A and Distler J. Progressive fibrosing interstitial lung disease associated with systemic autoimmune diseases. Clin Rheumatol. 2019;38(10):2673–2681.
-
Mathai SC and Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ. 2016;352:h6819.
-
Wallace B, Vummidi D, Khanna D. Management of connective tissue diseases associated interstitial lung disease: a review of the published literature. Curr Opin Rheumatol. 2016;28(3):236–245.
-
Spagnolo P, Cordier JF, Cottin V. Connective tissue diseases, multimorbidity and the ageing lung. Eur Respir J. 2016;47(5):1535–1558.
-
Vacchi C, Sebastiani M, Cassone G, et al. Therapeutic options for the treatment of interstitial lung disease related to connective tissue diseases. A narrative review. J Clin Med. 2020;9(2):407. doi: 10.3390/jcm9020407.
-
Koo SM, Kim SY, Choi SM, et al. Korean guidelines for diagnosis and management of interstitial lung diseases: part 5. Connective tissue disease associated interstitial lung disease. Tuberc Respir Dis (Seoul). 2019;82(4):285–297.
-
Doyle TJ, Hunninghake GM, Rosas IO. Subclinical Interstitial Lung Disease. Am J Respir Crit Care Med. 2012;185:1147–1153.
-
Flaherty KR, Brown KK, Wells AU, et al. Design of the PF-ILD trial: a double-blind, randomised, placebo-controlled phase III trial of ... in patients with progressive fibrosing interstitial lung disease. BMJ Open Resp Res. 2017;4(1):e000212.
-
Demedts M, Wells AU, AntÓ JM, et al. Interstitial lung diseases: an epidemiological overview. Eur Respir J Suppl. 2001;18(suppl32):2s-16s.
-
Cottin V, Hirani NA, Hotchkin DL, et al. Presentation, diagnosis and clinical course of the spectrum of progressive fibrosing interstitial lung diseases. Eur Respir Rev. 2018;27(150):180076. doi: 10.1183/16000617.0076-2018.
-
Spagnolo P, Distler O, Ryerson CJ, et al. Mechanisms of progressive fibrosis in connective tissue disease (CTD)-associated interstitial lung diseases (ILDs). Ann Rheum Dis. 2021;80:143–150.
-
Patterson KC, Strek ME. Pulmonary fibrosis in sarcoidosis. Clinical features and outcomes. Ann Am Thorac Soc. 2013;10(4):362-370.
-
Caban JJ, Yao J, Bagci U, Mollura DJ. Monitoring pulmonary fibrosis by fusing clinical, physiological, and computed tomography features. Conf Proc IEEE Eng Med Biol Soc. 2011;6216-6219.
-
Maher TM, Wuyts W. Management of fibrosing interstitial lung diseases. Adv Ther. 2019;36(7):1518–1531.
-
Assayag D, Lee JS, King Jr TE. Rheumatoid arthritis associated interstitial lung disease: a review. Medicina (B Aires). 2014;74(2):158–165.
-
Kawano-Dourado L, Doyle TJ, Bonfiglioli K, et al. Baseline characteristics and progression of a spectrum of interstitial lung abnormalities and disease in rheumatoid arthritis. Chest. 2020:S0012-3692(20)31412-4. doi: 10.1016/j.chest.2020.04.061.
-
Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27(2):269–281.
-
Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid Arthritis–Interstitial Lung Disease–associated Mortality. Am J Respir Crit Care Med. 2011;183:372–378.
-
Geerts S, Wuyts W, de Langhe E, et al. Connective tissue disease associated interstitial pneumonia: a challenge for both rheumatologists and pulmonologists. Sarcoidosis Vasc Dif. 2017;34:326–335.
-
Esposito AJ, Chu SG, Madan R, et al. Thoracic manifestations of rheumatoid arthritis. Clin Chest Med. 2019;40(3):545–560.
-
Shaw M, Collins BF, Ho LA, Raghu G. Rheumatoid arthritis-associated lung disease. Eur Respir Rev. 2015;24(135):1–16.
-
Kelly CA, Saravanan V, Nisar M, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics – a large multicentre UK study. Rheumatology (Oxford). 2014;53(9):1676–1682.
-
Bergamasco A, Hartmann N, Wallace L, et al. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clin Epidemiol. 2019;11:257–273.
-
Walker UA, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann Rheum Dis. 2007;66(6):754-763. doi:10.1136/ ard.2006.062901.
-
Alamanos Y, Tsifetaki N, Voulgari PV, et al. Epidemiology of primary Sjögren’s syndrome in north-west Greece, 1982–2003. Rheumatology. 2006;45:187–191.
-
Patel R, Shahane A. The epidemiology of Sjögren’s syndrome. Clin Epidemiol. 2014;6:247–255.
-
Watanabe M, Naniwa T, Hara M, et al. Pulmonary Manifestations in Sjögren’s Syndrome: Correlation Analysis Between Chest Computed Tomographic Findings and Clinical Subsets with Poor Prognosis in 80 Patients. J Rheumatol. 2010;37:365-373.
-
Sambataro G, Ferro F, Orlandi M, et al. Clinical, morphological features and prognostic factors associated with interstitial lung disease in primary Sjögren’s syndrome: A systematic review from the Italian Society of Rheumatology. Autoimmun Rev. 2020;19:102447.
-
Ramos-Casals M, P Brito-Zerón, Seror R, et al. Characterization of systemic disease in primary Sjögren’s syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology. 2015;54:2230-2238.
-
Roca F, Dominique S, Schmidt J, et al. Interstitial lung disease in primary Sjögren’s syndrome. 2016;http://dx.doi.org/10.1016/j.autrev.2016.09.017.
-
Cheeti A, Brent LH, Panginikkod S. Autoimmune Myopathies. [Updated 2020 Aug 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532860/. Last accessed Jan 2021.
-
Yang SH, Chang C, Lian Z-X, et al. Polymyositis and dermatomyositis – challenges in diagnosis and management. J Transl Autoimmun. 2019;2:100018.
-
Li R, Zhu W-J, Wang F, et al. AST/ALT ratio as a predictor of mortality and exacerbations of PM/DM-ILD in 1 year-a retrospective cohort study with 522 cases. Arthritis Res Ther. 2020;22(1):202.
-
Shappley C, Paik JJ, Saketkoo LA. Myositis-related interstitial lung diseases: diagnostic features, treatment, and complications. Curr Treatm Opt Rheumatol. 2019;5(1):56–83.
-
Fathi M, Vikgren J, Boijsen M, et al. Interstitial lung disease in polymyositis and dermatomyositis: longitudinal evaluation by pulmonary function and radiology. Arthritis Rheum. 2008;59(5):677–685.
-
Yu K, Wu YJ, Kyo C, et al. Survival analysis of patients with dermatomyositis and polymyositis: Analysis of 192 Chinese cases. Clin Rheumatol. 2011;30:1595-1601.
-
Ji S-Y, Zeng F-Q, Guo Q, et al. Predictive factors and unfavourable prognostic factors of interstitial lung disease in patients with polymyositis or dermatomyositis: a retrospective study. Chin Med J (Engl). 2010;123(5):517–522.
-
Chen I-J, Jan Wu Y-J, Lin C-W, et al. Interstitial lung disease in polymyositis and dermatomyositis. Clin Rheumatol. 2009;28(6):639–646.
-
Hayashi S, Tanaka M, Kobayashi H, et al. High-resolution computed tomography characterization of interstitial lung diseases in polymyositis/dermatomyositis. J Rheumatol. 2008;35(2):260–269.
-
Kang EH, Lee EB, Shin KC, et al. Interstitial lung disease in patients with polymyositis, dermatomyositis and amyopathic dermatomyositis. Rheumatology (Oxford). 2005;44(10):1282–1286.
-
Hoffmann-Vold AM, Fretheim H, Halse AK, et al. Tracking impact of interstitial lung disease in systemic sclerosis in a complete nationwide cohort. Am J Respir Crit Care Med. 2019;200;1258–1266.
-
Song JW, Lee H, Lee C, et al. Clinical Course and outcome of rheumatoid arthritis-related usual interstitial pneumonia. Sarcoidosis Vasc Dif. 2013;30:103–112.
-
Kolb M, Bondue B, Pesci A, et al. Acute exacerbations of progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018;27(150):pii:180071.
-
Suda T, Kaida Y, Nakamura Y, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Resp Med. 2009;103:846–853.
-
Cao M, Sheng J, Qiu X, et al. Acute exacerbations of fibrosing interstitial lung disease associated with connective tissue diseases: a population-based study. BMC Pulm Med. 2019;19:215.
-
Tomiyama F, Watanabe R, Ishii T, et al. High Prevalence of Acute Exacerbation of Interstitial Lung Disease in Japanese Patients with Systemic Sclerosis. Tohoku J Exp Med. 2016;239, 297–305.
-
Okamoto M, Fujimoto K, Sadohara J, et al. A retrospective cohort study of outcome in systemic sclerosis-associated interstitial lung disease. Respiratory Investigation. 2016;54, 445–453.
-
Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis – An International Working Group Report. Am J Respir Crit Care Med. 2016;194:265–275.
-
Song JW, Hong S-B, Lim C-M, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37(2):356–363.
Resources for patients




