Access to multi-disciplinary team (MDT) care built around an experienced MDT is vital for patients with chronic progressive fibrotic ILD
Multi-faceted care of individual patients with chronic progressive fibrotic ILDs
A diagnosis of chronic progressive fibrotic ILD can place a heavy burden on patients’ physical and emotional needs.5 Holistic approaches to ongoing management emphasise a role for anti-fibrotic treatment and supportive care individualised to the patient.1-4
Early supportive (palliative) care can help to relieve symptoms and maintain physical and emotional wellbeing in patients with chronic progressive fibrotic ILD.1 Pulmonary rehabilitation can significantly improve mobility and quality of life, as well as dyspnoea and depression scores. The benefits of pulmonary rehabilitation last for at least 6 months in most patients with interstitial lung disease (ILD).2
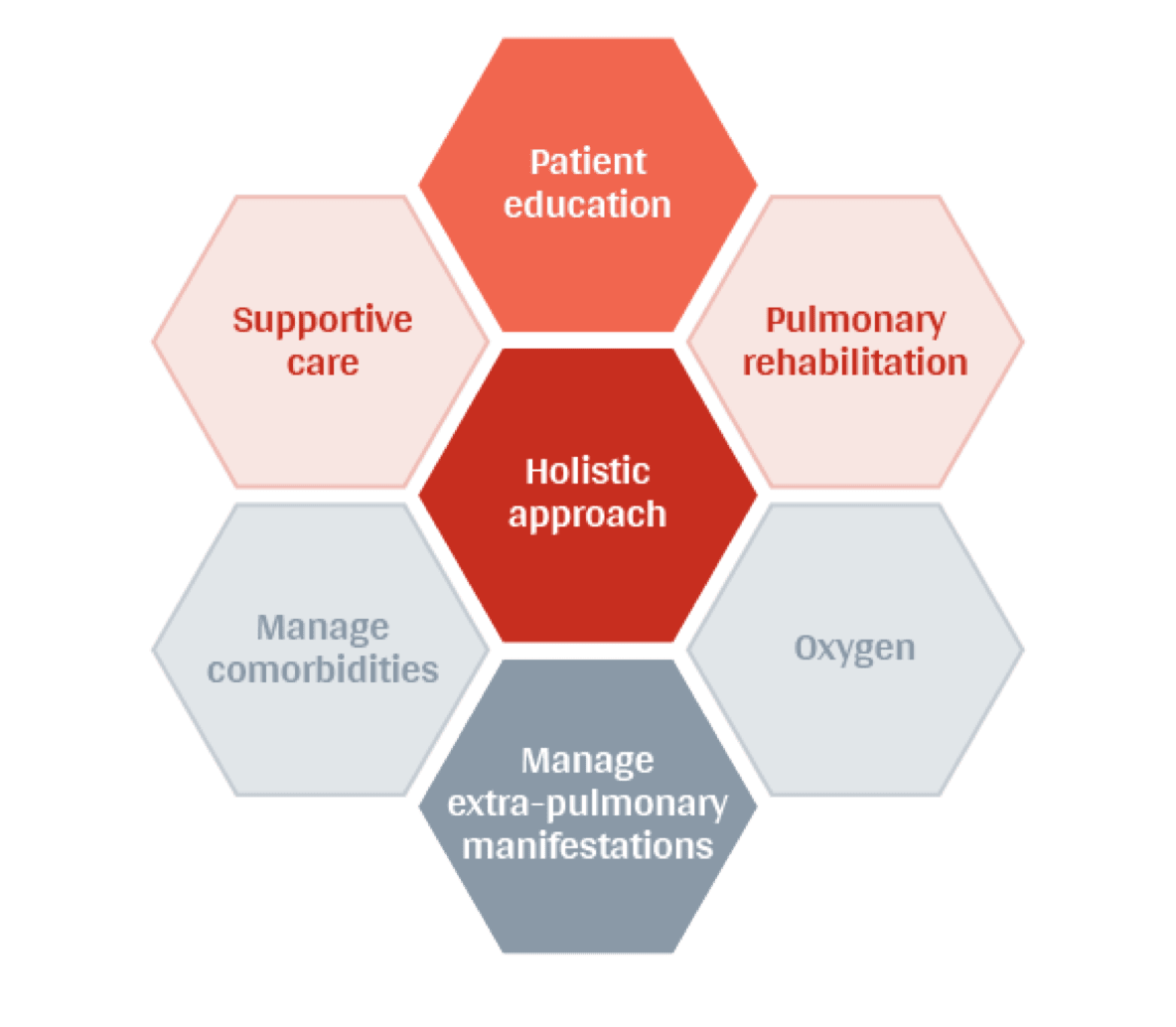
Chronic progressive fibrotic ILD patients need access to specialist care
ILD Specialist Centres are well-equipped to offer holistic care and may be able to provide access to a wide range of interventions, support material and specific MDT expertise. Through these centres chronic progressive fibrotic ILD patients can receive the best available care and may also be eligible to participate in clinical trial programmes.6

Licensed treatments such as OFEV® (nintedanib)

Early evaluation for lung transplantation

Pulmonary rehabilitation

Oxygen therapy

Support services and counselling
Avoiding exposure to potentially harmful inappropriate treatments
Action should be taken at the earliest suspicion of chronic progressive fibrotic ILD
Timely referral to a specialist ILD centre can help diagnose and appropriately treat ILDs to minimise the irreversible loss of lung function7,8

How to get patients where they need to be in the shortest possible time?
How can you extend the anti-fibrotic benefits of OFEV® in IPF to more patients with chronic progressive fibrotic ILD?
- Kreuter M et al. Lancet Respir Med 2017;5(12):968-980.
- Ryerson CJ et al. Respir Med 2014;108(1):203-210.
- Maher TM and Wuyts W. Adv Ther 2019;36:151811531.
- Sgalla G et al. BMJ Open Respir Res 2015;2(1):e000065.
- Swigris JJ and Kradin R. EXPLORE IPF: A Survey Evaluating Patient and Caregiver Perspectives in Idiopathic Pulmonary Fibrosis. Supplement to Chest Physician. 2015.
- Spagnolo P et al. Multidisc Respir Med 2012;7(1):42.
- Molina-Molina M et al. Exp Rev Resp Med 2018;12(7):537-539.
- Cottin V and Cordier VC. Eur Respir J 2012;40(3):519-521.
- OFEV® 100 mg and 150 mg soft capsules Summary of Product Characteristics. Boehringer Ingelheim.
Adverse events should be reported.
UK: Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Boehringer Ingelheim Drug Safety on 0800 328 1627 (freephone) or by email to PV_local_uk_ireland@boehringer-ingelheim.com.
IE: Reporting forms and information can be found at www.hpra.ie/homepage/about-us/report-an-issue. Adverse events should also be reported to Boehringer Ingelheim Drug Safety on 01 291 3960 or by email to PV_local_uk_ireland@boehringer-ingelheim.com.
OFEV® is indicated in adults for the treatment of idiopathic pulmonary fibrosis (IPF) and for the treatment of other chronic fibrosing interstitial lung diseases (ILDs) with a progressive phenotype. The recommended dose is one 150 mg capsule taken twice daily.9