Sitemap
- OFEV® 100 mg and 150 mg soft capsules Summary of Product Characteristics. Boehringer Ingelheim.
Adverse events should be reported.
UK: Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Boehringer Ingelheim Drug Safety on 0800 328 1627 (freephone) or by email to PV_local_uk_ireland@boehringer-ingelheim.com.
IE: Reporting forms and information can be found at www.hpra.ie/homepage/about-us/report-an-issue. Adverse events should also be reported to Boehringer Ingelheim Drug Safety on 01 291 3960 or by email to PV_local_uk_ireland@boehringer-ingelheim.com.
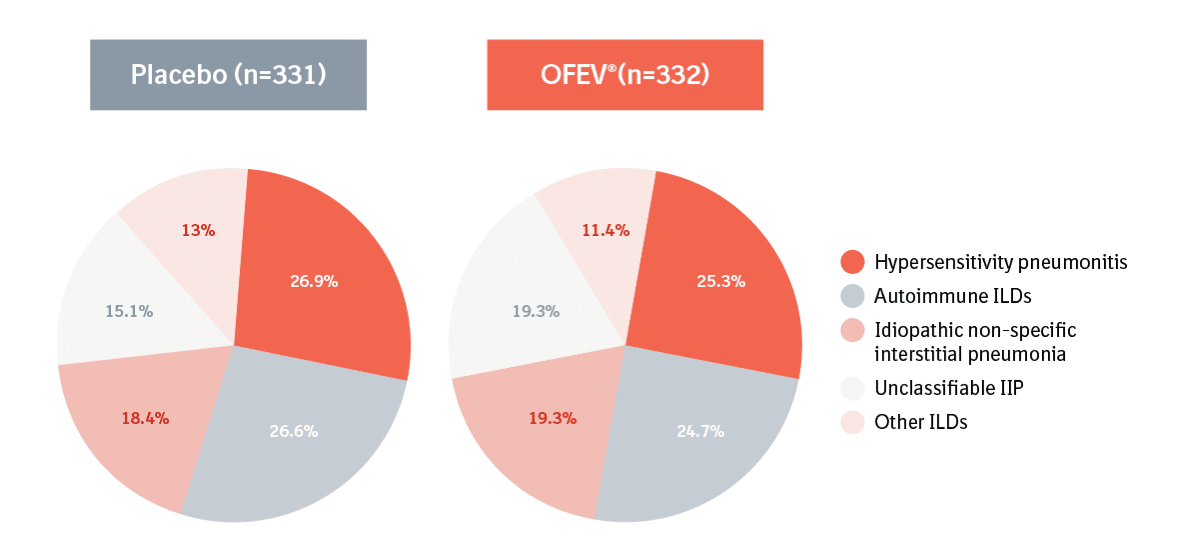
OFEV® is indicated in adults for the treatment of idiopathic pulmonary fibrosis (IPF) and for the treatment of other chronic fibrosing interstitial lung diseases (ILDs) with a progressive phenotype. The recommended dose is one 150 mg capsule taken twice daily.1