This library of practical tools and educational resources aims to support healthcare professionals in Ireland in delivering and optimising treatment and care for their pulmonary fibrosis patients.
Simple Dosing and Clear Monitoring

Nutrition booklet

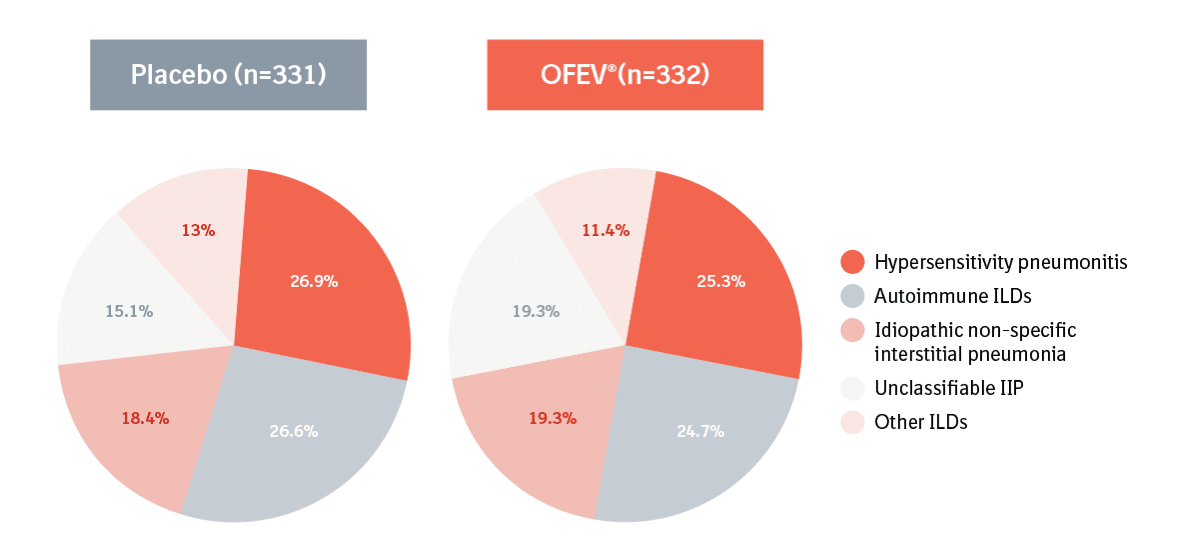
INBUILD® vs INPULSIS®

Ireland ILD Specialist Centres Map
Sounds of IPF

Progressive Pulmonary Fibrosis (PPF) expert videos
- OFEV® 100 mg and 150 mg soft capsules Summary of Product Characteristics. Boehringer Ingelheim.
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie/homepage/about-us/report-an-issue. Adverse events should also be reported to Boehringer Ingelheim Drug Safety on 01 291 3960 or by email to PV_local_uk_ireland@boehringer-ingelheim.com.
OFEV® (nintedanib) is indicated in adults for the treatment of idiopathic pulmonary fibrosis (IPF) and for the treatment of other chronic fibrosing interstitial lung diseases (ILDs) with a progressive phenotype. The recommended dose is one 150 mg capsule taken twice daily.1